“We now have a much more definitive test that gives us very specific answers on organisms we really didn't know existed in infected joints.”
Jon E. Minter, DO
Arthritis & Joint Specialists, Alpharetta, GA
Watch clinician testimonials
Diagnosing Challenging Orthopedic and General Surgery Infections
Orthopedic infections such as septic arthritis, periprosthetic joint infections (PJI), and osteomyelitis pose significant diagnostic and therapeutic challenges. These infections are often polymicrobial and involve biofilms on native or prosthetic joint surfaces, which shield pathogens from immune defenses and reduce antibiotic effectiveness. Prior antibiotic use and the growing issue of antimicrobial resistance make accurate diagnosis even more difficult.
Conventional cultures often fail to detect the full spectrum of pathogens, especially in biofilm-associated or low-abundance infections. Delayed or incomplete diagnosis increases the risk of chronic infection, implant failure, and poor surgical outcomes.
MicroGenDX provides rapid microbial DNA sequencing for improved orthopedic infection diagnostics and better clinical outcomes. OrthoKEY Outpatient and OrthoKEY Surgery are validated for synovial fluid, intraoperative swabs, tissue samples, and gauze to support the diagnosis of:
- Periprosthetic Joint Infections (PJIs)
- Septic arthritis
- Osteomyelitis
- Non-union trauma
- Spine infections
Powered by next-generation sequencing (NGS) and qPCR, our test offers high-sensitivity detection of bacterial and fungal pathogens, even in biofilm-associated or culture-negative infections. Resistance gene profiling enables targeted antimicrobial selection.
Our lab report provides orthopedic surgeons actionable microbiological data to support earlier diagnosis, optimize surgical planning, and improve infection management outcomes.
Clinical Insights from our Ortho and General Surgery Samples
Increased Sensitivity in Pathogen Detection:
Studies have demonstrated that NGS exhibits superior sensitivity compared to conventional culture methods. In a prospective study evaluating PJI, NGS identified infections in 25 of 28 cases that met the Musculoskeletal Infection Society (MSIS) criteria, whereas culture detected only 17 [1]
Identification of Culture-Negative Infections:
A multi-center study involving over 300 participants revealed that NGS detected potential pathogens in 66% of culture-negative PJI cases, with 91% of these cases demonstrating polymicrobial infections. [2]
Detection of Polymicrobial Etiologies:
NGS accurately identifies multiple pathogens within a single sample, unlike culture, which often fails in polymicrobial infections. In shoulder PJI, NGS identified multiple organisms in culture-positive cases, with over 90% classified as polymicrobial. [3]
Identification of Fastidious and Biofilm-Associated Microorganisms:
MicroGenDX NGS effectively detects slow-growing, anaerobic, and biofilm-embedded microbes that are often missed by traditional culture. A prospective study showed that 67% of unexpected failures by infection were attributed to untreated or undertreated pathogens previously detected by NGS but missed by culture. [4]
- 1. Tarabichi M., Shohat N., Goswami K.,. et al. Diagnosis of Periprosthetic Joint Infection - The Potential of NGS Journal of Bone & Joint Surgery, 2018
- 2. Goswami K., Clarkson J. S., et al. An Enhanced Understanding of Culture-Negative Periprosthetic Joint Infection with Next-Generation Sequencing: A Multicenter Study The Journal of Bone and Joint Surgery, 2022
- 3. Namdari S., Nicholson T., et al. Comparative study of cultures and next-generation sequencing in the diagnosis of shoulder prosthetic joint infections. Journal of Shoulder and Elbow Surgery, 2019
- 4. Rogalski L. B., Goswami K., et al. An Enhanced Understanding of Shoulder PJI using Next generation Sequencing: Findings at 3-year Clinical Follow-up. Journal of Shoulder and Elbow Surgery, 2022
Recommended use of Next-Generation DNA Sequencing in Orthopedics

Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2024 Update by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM).
For joint infections "16S ribosomal RNA gene PCR/sequencing or a multiplex PCR panel may be considered if cultures are negative."

International Consesus Meeting 2018 (Philadelphia)
“The consensus highlighted the potential of NGS to identify pathogens in complex infections where traditional culture methods might fail, making it a valuable tool in orthopedic settings.”
International Consesus Meeting 2025 (Istanbul)
92% of delegates endorse molecular diagnostics as promising adjunct to conventional methods to diagnose PJI and isolating infecting organisms, in particular for:
- Culture negative infections
- Rapid detection of pathogens
- Suspicion of rare pathogens
- High risk patients
“MicroGenDX will give you quantitative and qualitative information regarding what organisms are present in a way that can actually guide you towards a clinical decision.”
Javad Parvizi, MD, FRCS
Orthopaedic Surgeon; Director of Clinical Research Rothman Orthopaedic Institute
Watch clinician testimonials
Interpreting our lab report
Each test includes a detailed laboratory report, providing clinicians with clear, structured data for fast decision-making.
These reports include:
• List of bacteria and fungi detected in the sample(s)
• Identified antibiotic resistance genes
• Antimicrobials to consideration for treatment



OrthoKEY Outpatient
Orthopedic Infection Test (synovial aspiration)
Synovial Fluid, Swab
NGS DNA + qPCR Diagnostics for accurate microbial identification
17 antibiotic resistance genes & list of antimicrobials for consideration
3-5 Business days (excludes transit)


OrthoKEY Surgery
Orthopedic Infection Test (surgical applications)
Synovial Fluid, Swab, Tissue, Gauze
NGS DNA + qPCR Diagnostics for accurate microbial identification
17 antibiotic resistance genes & list of antimicrobials for consideration
3-5 Business days (excludes transit)


SurgeryKEY-Test
Surgical Site Infection Test
Swab, Tissue, Fluid
NGS DNA + qPCR Diagnostics for accurate microbial identification
17 antibiotic resistance genes & list of antimicrobials for consideration
3-5 Business days (excludes transit)
Publications Demonstrating the impact of NGS in Orthopedics
Next-Generation Sequencing in Periprosthetic Joint Infections: Clinicians’ Guide to Its Diagnostic Role
Kullar R, Tipton CD, File TM, Shahi A, Sniffen JC, Goldstein EJC. Infect Dis Clin Pract. 2025
Review: This recent review, published in May 2025, outlines the emerging role of next-generation sequencing (NGS) in diagnosing periprosthetic joint infections (PJI), particularly in cases where conventional cultures fail—such as chronic or biofilm-associated infections. It summarizes current insights on diagnostic accuracy and clinical application.
The authors highlight that NGS can identify a broader spectrum of pathogens, including fastidious and polymicrobial organisms, directly from clinical samples. This is especially valuable in culture-negative PJIs, where treatment decisions often lack microbiological confirmation.
Clinically, the review supports NGS as a complementary tool that improves pathogen detection and enables earlier, targeted antimicrobial therapy ultimately enhancing surgical planning and outcomes.

An Enhanced Understanding of Culture-Negative Periprosthetic Joint Infection with Next-Generation Sequencing: A Multicenter Study
Goswami K, Clarkson S, Phillips CD, et al. The Journal of Bone and Joint Surgery, 2022
Study: This multicenter study investigated culture-negative periprosthetic joint infections (PJI) using next-generation sequencing (NGS) to better characterize their microbial profiles. Traditional culture methods often fail to identify causative pathogens, leaving treatment decisions uncertain.
Using NGS, the authors found that nearly two-thirds of culture-negative PJIs contained opportunistic pathogens. Notably, most infections were polymicrobial, underscoring the complexity of these cases.
These findings demonstrate that NGS can uncover clinically relevant organisms in culture-negative PJIs, offering a more complete microbial picture and enabling more precise, targeted treatment strategies.

Next-Generation Sequencing Supports Targeted Antibiotic Treatment for Culture Negative Orthopedic Infections
Kullar R, Chisari E, Snyder J, Cooper C, Parvizi J, Sniffen J. Clinical Infectious Disease, 2022
Review: This review, authored by experts in infectious diseases, orthopedics, and microbiology, addresses the challenge of diagnosing culture-negative orthopedic infections, where traditional methods often fail to detect causative pathogens. Such limitations can delay appropriate therapy and complicate clinical management.
The authors highlight how next-generation sequencing (NGS) improves pathogen detection, especially when used early and with supporting clinical criteria. By identifying difficult-to-culture organisms, NGS enables targeted antibiotic treatment, reduces reliance on empiric therapy, and supports more precise infection management.

Current Recommendations for the Diagnosis of Acute and Chronic PJI for Hip and Knee—Cell Counts, Alpha-Defensin, Leukocyte Esterase, Next-generation Sequencing
Goswami K, Parvizi J, Courtney PM.Current Reviews in Musculoskeletal Medicine, 2018
Review: This review outlines the persistent challenges in diagnosing acute and chronic periprosthetic joint infections (PJI), emphasizing that no single test provides definitive accuracy. Current approaches rely on a combination of serological, synovial, microbiological, histological, and imaging tools - many of which are invasive, costly, or insufficiently sensitive.
Emerging diagnostics such as synovial biomarkers and molecular methods offer improved detection capabilities. Among these, next-generation sequencing (NGS) is particularly promising, as it can identify a broad range of pathogens, including those missed by culture, and is especially valuable in complex or culture-negative cases.
The review concludes that NGS enhances diagnostic precision by revealing hidden infections and guiding more personalized antimicrobial strategies - supporting its role as a valuable adjunct in PJI evaluation.

Clinical cases where MicroGenDX test helped achieve treatment success
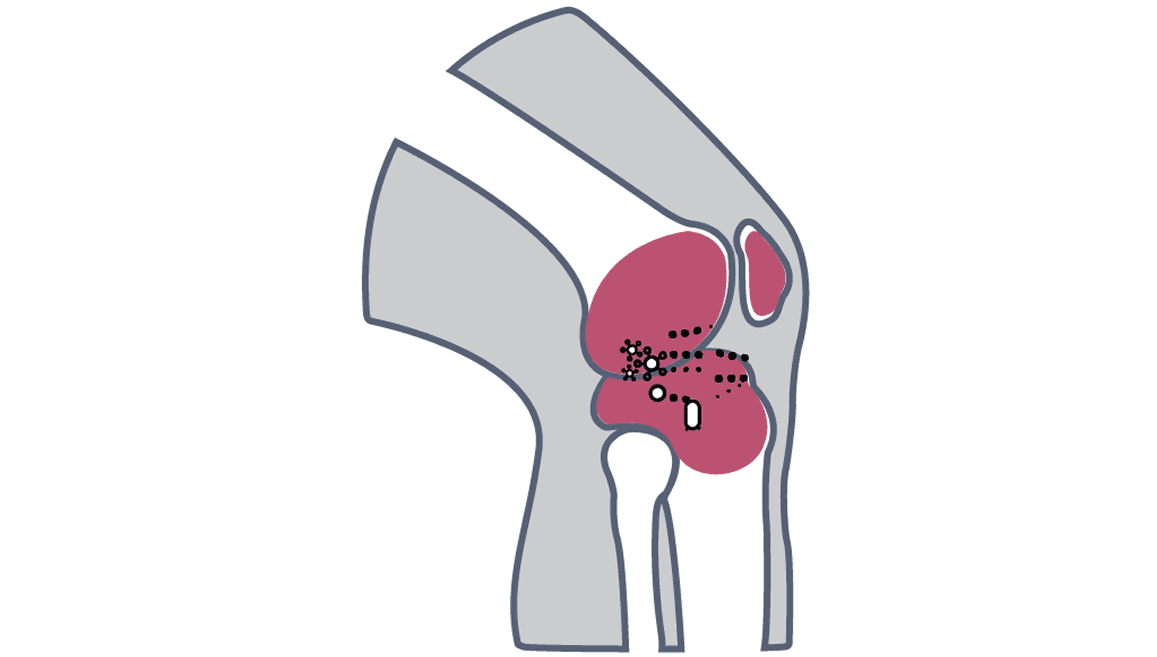
NGS identifies Kocuria missed by MALDI-TOF in joint infection
A 77-year-old male with history of type 2 diabetes mellitus, hypertension, and hyperlipidemia presented to clinic with pain and stiffness to his right knee. Surgical history included a right TKA and subsequent revision surgery. Biomarkers from synovial fluid and serum were consistent with infection.
Synovial fluid and intraoperative samples were sent for culture MALDI-TOF and MicroGenDX NGS identification. Culture MALDI-TOF isolated very light growth Staphylococcus spp. and gram positive cocci. However, MicroGenDx NGS identified Kocuria ocularis to be the main culprit. A research MALDI-TOF database confirmed NGS identification of Kocuria ocularis. Patient treatment changed from vancomycin to penicillin, and patient was recovering well at 6-month follow-up.
The reference database used for MALDI-TOF did not contain Kocuria ocularis. An inability to identify an organism in this manner, along with the morphologic similarity to some coagulase-negative Staphylococcus (CoNS) species, led to the inaccurate reporting of the sample and initial implementation of incorrect antibiotic treatment.
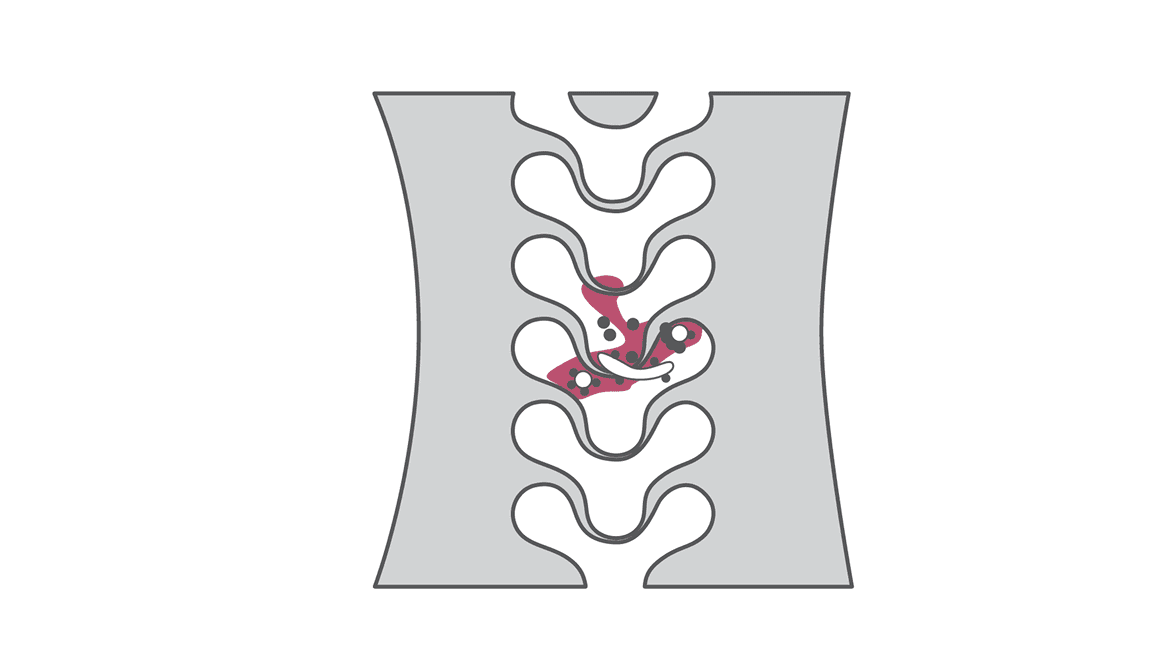
NGS quickly identifies Mycobacterium smegmatis in spine implant infection
A 62-year-old female with history of osteoarthritis, hyperlipidemia, depression, and anxiety initially presented to clinic with approximately two years of frequent falls and progressively worsening issues with balance and coordination. She denied any neck or back pain, but did note weakness, numbness, and tingling to upper extremities, with symptoms worse on the right side. She had begun to require the use of a walker for ambulation, and saw no relief with multiple epidural injections or aqua therapy. Physical exam revealed decreased range of motion of her cervical spine, and decreased strength to bilateral triceps, wrist flexors, and intrinsics. CT and MRI of the cervical spine demonstrated myelopathy and destructive changes consistent with advanced osteoarthritis at C1-C2, and the patient subsequently underwent a C1 to C3 laminectomy and occiput to C5 posterior fusion.
At one-month follow-up, the patient reported intermittent posterior neck pain and frontal headaches, but overall her symptoms had improved. However, at 42 days status-post her initial surgery, she presented to clinic with a week of shooting pain up her posterior head, and radiographic imaging revealed two occipital screws displaced and migrated from the plate. As the patient showed no constitutional signs or symptoms of infection, she was indicated for a revision surgery with placement of new instrumentation. However, during the procedure, there was noted to be a large amount of inflammatory tissue surrounding the disengaged screws, and three samples of this tissue were sent for analysis with culture.
Culture of tissue grew a nontuberculous mycobacteria (NTM) in five days, but a specific species was not identified using culture until five weeks following sample collection. In contrast, next-generation sequencing (NGS) analysis performed on samples obtained during a subsequent irrigation and debridement confirmed the presence of Mycobacterium smegmatis within one week. Although traditional culture identified a rapidly growing NTM in this case of spine implant infection (SII), the species was not identified in a timely manner. Accurate and rapid species-level pathogen identification is imperative in the targeted treatment of spinal infections including those caused by NTM. The use of molecular testing such as NGS is helpful in identifying infecting organisms and guiding antimicrobial treatment.
Inquire about our test
Send us a message using the form below, or email us at info@microgendx.eu
